|
Marine crisis looms over acidifying oceans NewScientist : 30 June 2005 The oceans are gradually turning into a vast 'fizzy drink', a transformation that could be catastrophic for ocean life. Levels of carbonic acid - the reaction product of water and carbon dioxide that is found in soda water - are increasing at a rate one hundred times faster than the world has seen for millions of years. The cause is the increasing level of CO2 in the atmosphere. These are the conclusions of the first review about the acidification of the oceans. The report was produced by an international group of scientists, commissioned by the Royal Society, the UK's national academy of science.
|
|
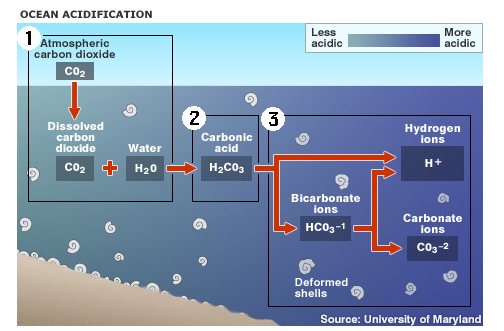
1. Up to one half of the carbon dioxide (CO2) released by burning fossil fuels over the past 200 years has been absorbed by the world's oceans 2. Absorbed CO2 in seawater (H2O) forms carbonic acid (H2CO3), lowering the water's pH level and making it more acidic 3. This raises the hydrogen ion concentration in the water, and limits organisms' access to carbonate ions, which are needed to form hard parts. |
|
The oceans are naturally alkaline but, since the industrial revolution, the sea surfaces have been turning ever more acidic. If CO2 emissions continue at current rates then by 2100 the pH of the sea will fall by as much as 0.5 units from its current level of pH 8.2. Such a change would be effectively irreversible. 'It will take many thousands of years for natural processes to return the oceans to their pre-industrial state,' says John Raven, at the University of Dundee, UK.
The sea life expected to be worst hit include organisms that produce calcium carbonate shells, as these are harder to form in acidic waters. That means that corals, molluscs and certain plankton species will be at risk. And it does not stop there. There is an important group of photosynthetic plankton that grow calcium carbonate shells and form giant blooms in spring and summer before sinking to the bottom of the ocean. But the increasing acidification will hinder their ability to grow, meaning they remove less carbon from the atmosphere. This in turn will result in more carbonic acid being formed at the seas' surface. 'Calcium carbonate helps organisms to sink and enhances the biological pump,' says Andrew Watson, an environmental biologist at the University of East Anglia, UK. The sea has absorbed about half of the CO2 produced by humans in the last 200 years and currently takes up one tonne of the gas each year for every person on the planet. But if the water becomes too acidic, the pump will not work and the ability of the oceans to mop up CO2 will fall, he says. Back |