|
From atomos to strings
Evolution of our understanding of matter |
|
Prologue An important part which distinguishes the modern world from the ancient is the supremacy of the mind. This novel idea first arose in Greece during the sixth century BC. That was in stark contrast to the rest of the ancient world - dominated by pharaos and king-priests - where magical forces ruled the minds of most people. In a world where the irrational and mystical played a major role, the Greeks came forward as the protagonists of the rational mind. |
|
600 BC In Ionia inquiring Greeks first began to speculate what the real nature of the world was and what its destiny might be. This analysis was called philosophia, or 'love of wisdom', and included what we today distinguish as philosophy and science. ( Destiny exercised a great influence on all Greek thought and might be one of the sources from which science derived the belief in natural law ). |
| Greek philosophers began to investigate the laws of nature, and they became essentially the first scientists. |
|
Thales of Miletus, the earliest of the Ionian philosophers, initiated the revolutionary notion that to understand
the world one needed to know its nature ( 'physis', hence the modern 'physics' ) and that there
was an explanation for all phenomena in natural terms. That was a giant step from the assumptions of the old world that supernatural forces determined almost everything. |

|

|
460 BC Democritus, from Greece, developed the concept of dividing matter into smaller and smaller pieces until you could divide it no more. He called these smallest pieces atoms ( atomos = indivisible in Greek ). This was a philosophical idea rather than a scientifically based theory. |
| After the Greek era scientific investigations greatly diminished. The Romans, although great engineers, were less interested in the nature of matter. And with the onset of the Middle Ages dogma became more important than science. |
|
As a result - for over two thousand years - the atomic theory lay dormant. |
|
1803 - atomic theory John Dalton, English chemist, revived the term of the atom when he suggested that each element was made up of unique atoms and the atoms of an element are all the same. He formulated his theory that chemical reactions result from the union and separation of these atoms and that atoms have characteristic properties. Combinations of atoms bound to each other he designated as molecules. |

|
|
1869 - periodic table D. I. Mendeleyev, Russian chemist, developed the periodic law of the properties of the chemical elements. * This law states that elements show a periodicity (regular pattern) of properties when they are arranged according to atomic weight. Several new elements were found in nature, and their chemical behavior exactly matched that predicted by Mendeleyev's periodic table. |

|
|
* element = The simplest type of chemical substance. Elements can be thought of as fundamental in chemical reactions. They cannot be made any simpler using chemical reactions.
For a while it seemed that atoms were fundamental. As their name suggests, they could not be split into anything simpler. |
|
However, towards the end of the 19th century, it became clear that atoms are not fundamental - they themselves are made of smaller particles. One of these atomic particles is the electron, which we now think is fundamental.
1897 - the electron The English physicist J.J. Thompson discovered a sub atomic particle common to all matter while investigating thermionic emissions. These emissions occur when a heated metal element releases an invisible beam. 
This beam can be fired at a a fluorescent screen by placing a small electrode a few centimetres away from the heated element and connecting a voltage between the element and the electrode. The electrode has to be positive (anode) and the element has to be negative (cathode). * Because the beam is attracted to the anode, Thomson deduced that cathode rays were a stream of negative particles. He called these electrons. This suggests that electrons have been released from the metal atoms. Later, it was shown that all atoms contain electrons i.e. the electron is one of the building blocks of a non-fundamental atom. |
| * The positive designation was an arbitrary choice following on earlier research showing that there were two kinds of electrical charge, like repelling and attracting the other type. |
|
. 1911 - the nucleus Ernest Rutherford bombarded gold foil with helium nuclei ( alpha particles ) and noticed that most go through the gold undeflected. But approximately 1 in 8000 were perceptibly deflected into all directions and some even bounced back in the direction they came. He concluded that these occasional deflections required that the gold atom contained a tiny positively charged center surrounded by negatively charged particles and consequently that matter is mostly empty space. |
 ( Graphics from : http://www.chemsoc.org/timeline/pages/timeline.html ) |
|
Rutherford worked out that the diameter of the nucleus is about 100,000 times smaller than the atom.
We can think of it as being like a pinhead in the middle of a soccer stadium where the pinhead would be the nucleus.
( Rutherford 's work gave the first indication
that the positive charge centre occupied an extremly small fraction of an atom's volume. )
After the discovery of the electron, it was realized that there must be positive charge centers within the atom to balance the negative electrons and create electrically neutral atoms. Rutherford's experiments convinced him that the hydrogen nucleus (being the lightest of all nuclei) was an elementary particle. He named it the proton , from the Greek word 'protos', meaning 'first'. He also predicted the existence of the neutron in 1920. Twelve years later, his assistant James Chadwick found it.
However, protons and neutrons are all attracted to each other as a result of another force - the strong nuclear force. This is an attractive force that only has an effect over a very short range in the nucleus. The strong nuclear force binds protons & neutrons together to make the nucleus. The neutrons don't contribute any repulsive effects because they are neutral. So having more neutrons around can help to hold the nucleus together. |
|
It was pointed out that any atom with electrons in orbit around a central positive charge should
continuously give out electromagnetic waves and therefore continuously lose energy, causing the electron
to spiral into the nucleus.
To avoid this Niels Bohr, from Denmark, simply postulated distinct permissible orbits as a new rule. |
|
1912 Niels Bohr suggested that the electrons orbiting the nucleus of atoms can only have certain discrete energies and that each element had different electron energies. He assumed that the orbits were defined by their angular momentum. This led to calculation of possible energy levels for these orbits and the postulation that the emission of light occurs when an electron moves into a lower energy orbit. |

|
|
This postulation rested on Einstein's proposition that energy, in the form of light, consists of distinct,
discontinuous particles (or quanta), which he called photons.
In Bohr's model of the atom, electrons could only occupy particular orbits around the nucleus, and they could jump from one orbit to the next - the famous quantum leap. With each such leap, they would emit or absorb a photon. Although the quantum theory of light was experimentally proven, other experiments had proven that light had also continous wavelike properties. Einstein suggested that there were "two contradictionary pictures of reality; separately neither of them fully explains the phenomena of light, but together they do". That meant that phenomena in nature could partake of both the wave and the particle, that light was at once both continuous and discrete. |
|
1924 In response to that difficulty Louis deBroglie , from France, suggested that matter, like light, has the properties of both particles and waves. This particle-wave duality - derived from the work of Einstein and Planck - was experimentally confirmed, for the electron, in 1927.
1925 - wave mechanics |
|
A new theory of physics, called Quantum Mechanics, presented an even more radical picture of the atom. |
| Max Born argued that Schrodinger's waves had nothing to do with material reality at all. Actually, light and matter exist as particles; what behaves like a wave is the probability of where that particle will be. The reason light sometimes appears to act as a wave is because we are noticing the accumulation of many of the light particles distributed over the probabilities of where each particle could be. |
|
1926 German physicist Werner Heisenberg formulated his uncertainty principle which says that you cannot know by measurement the position and momentum of a particle simultaneously. The better you know one, the worse you know the other. Such uncertain aspects of the microscopic world become ever more severe, as the distance and time scales on which they are considered become ever smaller. |

|
|
Particles and fields undulate and jump between all possible values consistent
with the quantum uncertainty. This implies that the microscopic realm is a
rolling frenzy, awash in a violent sea of quantum fluctuations. Quantum fluctuation : turbulent behavior of a system on microscopic scales due to the uncertainty principle. Atoms were now visualized as a nucleus surrounded by a cloud of electrons distributed according to a wave pattern by the Schrodinger equation. Schroedinger equation : equation governing the evolution of probability waves in quantum mechanics. |
|
|
This cloud- chamber photograph (1932) shows the track of a positively charged particle of electronic mass slowed down by passing upward through a lead plate. It was among the earliest evidence of the existence of the positron. |

|
| The introduction of probability constituted a radical break with traditional physics which had contained as a fundamental principle the categorical link of cause and effect. |
|
The probabilistic nature of quantum mechanics meant that sometimes events happen for no discernable reason.
Or, more correctly, events happen due to comprehensible and predictable underlying causes, but at unpredictable times.
It was now no longer possible to link consequent events - the effects - to any cause that would allow to predict what would happen next. Although consequent events can be predicted in terms of known interactions or forces, specific event times, or specific event choices when various possibilities compete, cannot be predicted except on a probabilistic basis. Reality would now be described via mathematical probabilities instead of images we could visualize.
The strangness of quantum mechanics led
Bohr to say: |
|
Inspite of strange wave mechanics and uncertainties (in terms of measurements) the basic fundamental building blocks of matter were now firmly established, or so it seemed for a time.
But then - in the second half of the 20th century - everything became complicated again. |
|
After 1945 scientists discovered hundreds of new particles with the help of new and more powerfull machines - particle accelerators - which could bombard sub-atomic material with much more force and at higher speed.
The results were absolutely stunning : |
|
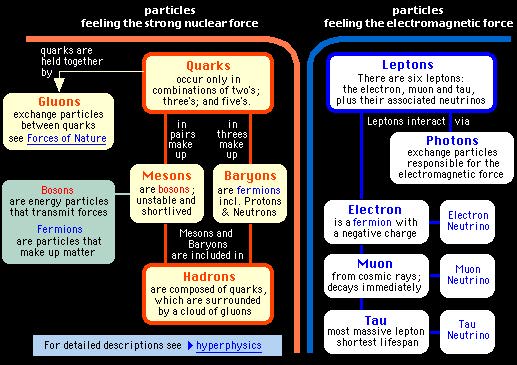
Where it had once seemed that there were a few different types of atomic constituent particles, suddenly there were nearly a hundred. Most of these particles were designated as hadrons. This is the family of particles that feel the strong nuclear force and includes protons and neutrons. ( The strong nuclear force holds protons and neutrons together in the nucleus ) However, apart from protons and neutrons, all the hadrons are very unstable and typically decay within a millionth of a second, and do not form part of normal matter. |

|
A new idea : Quarks
1960's The strong nuclear force is actually a force between quarks and is carried by particles called gluons (very much analogous to photons being the carrier of elechtromagnetic forces). Protons and neutrons are made of quarks and they feel the strong nuclear force as well. |
|
Besides the strong nuclear force we also recognize the distinctly different |
| Electrons do not feel the strong nuclear force. Fundamental particles that don't feel the strong nuclear force are all in the family of leptons. |
| Another examples of a lepton is the neutrino (meaning little neutral one). The neutrino wasn't found in an experiment until 1957. This is because the forces between it and other particles are extremely weak. It is thought that a neutrino can pass through the Earth with only a 1 in 200 million chance of interacting with any other matter. |
| The leptons and quarks are different from each other, most notably : |
|
Leptons can exist on their own whereas quarks only exist in combination with other quarks making hadrons.
Quarks feel the strong nuclear force and leptons do not. |
 Quarks only exist inside hadrons because they are confined by the strong force fields. Therefore, we cannot measure their mass by isolating them. The nature of the strong force between quarks does not permit isolated individual quarks to exist.
Quarks only exist inside hadrons because they are confined by the strong force fields. Therefore, we cannot measure their mass by isolating them. The nature of the strong force between quarks does not permit isolated individual quarks to exist.
This is a radically new feature of the strong force, never before encountered, but understandable in terms of the details of the strong forces characteristics. The table below explains the Quark and Lepton families. |
|
|
The Standard Model
is a theory that explains ( for now ) what matter consists of and what holds everything together.
In the Standard Model, there are 12 fundamental particles : The Universe is made up of combinations of these particles and all normal matter is made from just three of these particles. Protons and neutrons are made of two types of quark. Physicists differentiate quarks according to charges specified by the strong force interactions flavour. Each quark has a different flavour, typically the types of charge being given convenient names such as up, or down. Physicists usually refer to them in three generations of pairs : |
|
up and down strange and charm top and bottom |
First generation Second generation Third generation |
|
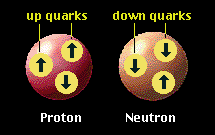
Only two flavours of quark are needed to make protons and neutrons: up and down.
The up & down quarks are the only quarks found in normal matter and they are known as the first generation. A proton is made of two up quarks and a down quark. A neutron is made from two down quarks and an up quark. |
|
|
Recapitulation 1) There are two families of fundamental particles - leptons and quarks. 2) Each family has three generations with two particles and two anti-particles.
3) All of normal matter is made from the first generations of these families. |
| The difficulty with the Standard Model |
|
Although physicists are now looking beyond the Standard Model, it still forms the basis of our understanding of the structure of matter. But the Standard Model is not a complete theory. It explains much of the structure of sub-atomic particles. However, it does not include the force of gravity and does not encompass the General Theory of Relativity.
Einstein spent the last thirty years of his life to find a Unified Field Theory, but the quest to find a unifying theory, which would combine all forces (that is the nuclear forces plus gravity) seemed to have forgotten with Einstein's death. But then interest suddenly revived, and part of the renewed interest came from astronomy, namely the discovery and research of Black Holes in the distant reaches of the Universe. |
| Black Holes are gigantic masses of matter collapsed into a tiny ball with a gravitational force so strong that even light cannot escape. The powerfull gravitional forces can only be investigated with Relativity, while the crushed nucleus of the Black Holes required Quantum Mechanics for answers. But the two theories were incompatible with each other. Physicists were therefore searching for new ideas. |
| NEXT : String Theories |
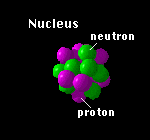 The neutrons can be thought of as acting as a kind of glue to hold the nucleus together. The positively charged protons repel each other.
The neutrons can be thought of as acting as a kind of glue to hold the nucleus together. The positively charged protons repel each other.
 Austrian physicist Erwin Schrodinger formed a model of a complete atom as interacting waves. The particles became like vibrations on a violin string, only they were closed in circles. His partial differential equation seemed to bear a similar
relation to the mechanics of the atom as Newton's equations of motion bear to planetary astronomy.
Austrian physicist Erwin Schrodinger formed a model of a complete atom as interacting waves. The particles became like vibrations on a violin string, only they were closed in circles. His partial differential equation seemed to bear a similar
relation to the mechanics of the atom as Newton's equations of motion bear to planetary astronomy.
